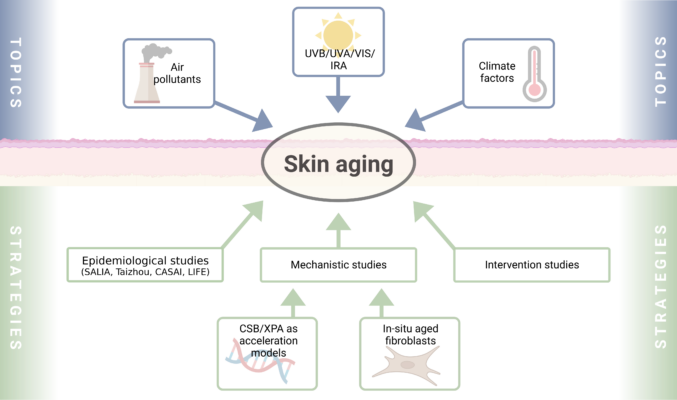
The Krutmann working group investigates the molecular mechanisms that underlie environmentally-induced skin aging and associated skin diseases. Major discoveries include: (1) the observation that the AHR is critically involved in the UVB stress response; (2) a potentially curative therapeutic approach for the UV-sensitive progeroid Cockayne syndrome; (3) the identification of traffic-related air pollution as a causative factor for skin aging/pigmentation; (4) the identification of near infrared rays as causative for skin wrinkle formation; (5) the definition and characterization of the skin aging exposome; (6) together with Klaus Unfried the development of an ectoine-based medical product for the protection against particle-induced lunge diseases; (7) the identification of two cutotypes in the skin microbiome of Han Chinese. J. Krutmann has regularly coordinated structured research consortia such as RTG 1033, SFB 728 and LRA Healthy Ageing. He currently serves as speaker of the DFG FOR 5489.

Head of working group:
Jean Krutmann
More than nuclear DNA damage – the role of mitochondria and metabolic alterations in the Nucleotide Excision Repair disorders Xeroderma pigmentosum and Cockayne syndrome
Within this project we aim to identify novel molecular mechanisms contributing to the UV hypersensitivity and DNA repair deficiency-associated diseases Xeroderma pigmentosum (XP) and Cockayne syndrome (CS) with particular emphasis on the involvement of mitochondria. We could previously show that the CSB protein is present at the centrosome where it interacts with proteins involved in acetylation of α-Tubulin, thereby promoting autophagy to prevent loss of subcutaneous fat, a hallmark of CS. Based upon these findings, we developed a pharmacological strategy to protect the subcutaneous fat in a mouse model of CS. With regard to XP, recent findings of our lab indicate that the Xeroderma pigmentosum type A (XPA) protein is located in the mitochondria and that its loss perturbs cellular energy metabolism and epigenetic regulation. We are currently investigating the function of mitochondrial XPA and the molecular mechanisms causing metabolic and epigenetic dysregulation. As both diseases, CS and XPA, do not only share skin symptoms but also a severe degenerative brain phenotype, we are now developing and characterizing human brain and skin organoids derived from iPS cells carrying disease-associated mutations in collaboration with the core unit GEMD.
Control of skin homeostasis by keratinocytes dependent on UVB-inducible PAS family transcription factors HIF-1a and AHR
UVB irradiation as a major environmental factor impacting the skin induces DNA lesions. Adaptative mechanisms of the skin to cope with UVB comprise tanning and acanthosis. UVB-induced immunosuppression frequently ameliorates chronic inflammatory skin disease but also increases skin cancer risk. Epidermal keratinocytes interact with surrounding cell types like melanocytes, Langerhans cells and T cells. The transcription factors HIF-1a (Hypoxia-inducible factor 1a) and AHR (Aryl hydrocarbon receptor) are both responsive to UVB radiation in keratinocytes, thus act as environmental sensors. They share the same co-factor and through different target genes, both modulate immune and repair responses to UVB irradiation. Yet, their interaction is scarcely studied. We investigate the combined function of HIF-1a and AhR in keratinocytes for skin homeostasis and during immune responses with the help of genetically modified in vivo models and Crispr/CAS9-mutated cell lines. We found that the skin of mice with HIF-1a-AHR-double-deficient keratinocytes phenotypically resembled UVB-irradiated (wildtype) animals. This raised the question whether their skin exhibits pre-existing improved protection against UVB or is in a state of increased stress. We also assess whether their cutaneous immune defense is disturbed similarly to UVB-induced immunosuppression of the wildtype and aim to identify underlying intracellular processes. These questions are being addressed through skin single cell transcriptomics, in vivo immune activation, and cell culture assays. In this project, we are closely collaborating with WGs Esser, Haarmann-Stemmann and the GEMD Core Unit. The project is funded by the DFG (DFG grant FA1468/2-2, follow-up funding of FA1468/2-1).
DFG FOR5489: P6 - Crosstalk between AHR signaling and retinoids in skin
Aryl hydrocarbon receptor (AHR) signaling is critical for skin barrier and photocarcinogenesis - both is also affected by retinoids (vitamin A derivatives). Their most relevant bioactive form in the skin is all-trans retinoic acid (atRA). atRA is metabolized from retinal by aldehyde dehydrogenase 1A enzymes. Besides endogenous atRA metabolism in keratinocytes, synthetic retinoids are being applied topically for therapeutic/cosmetic indications. There is compelling evidence from other tissues, that retinoid signaling and AHR signaling can influence each other after application of the permanent AHR activation dioxin (TCDD). It is currently not known how transient activation by clinically relevant AHR ligands affects retinoid metabolism, how retinoids impact AHR signaling caused by such ligands, and how these interactions might be relevant for skin. We observed that AHR signaling regulates atRA synthesis in human keratinocytes and murine skin and that a presumably AHR-dependent aldehyde dehydrogenase 1A isoform may be critically involved in this context. In addition, independent evidence suggests that a combined treatment of murine skin with atRA plus TCDD caused a proinflammatory, psoriasis-like skin response. We therefore hypothesize that in skin, AHR signaling and retinoid signaling influence each other, that this interaction is bidirectional and of clinical relevance, e.g. for skin barrier function. Specifically, we will assess AHR ligand-mediated regulation of atRA synthesis in keratinocytes, the role of the putatively AHR-dependent aldehyde dehydrogenase 1A isoform, and the relevance of endogenous retinoid synthesis as an integral part of AHR responses in keratinocytes. Further, we will assess the mechanistic crosstalk and functional outcome of exogenously added atRA and AHR ligands in skin. We will address these topics by employing different in vitro and in vivo approaches, modern genome engineering methods and bioinformatic analysis of big systemic data sets. In the context of this project as a part of the DFG research unit “Understanding aryl hydrocarbon receptor signaling in skin disorders”, we cooperate with numerous internal and external project partners.
Research training group (RTG) 2624: Biostatistical methods for high-dimensional data in toxicology
The research training group (RTG) 2624 is funded by the DFG (German Research Foundation) and the time period of the first phase is 04/2021-09/2025. Participating institutions together with the IUF are the Heinrich Heine University Düsseldorf, IfADo – Leibniz Research Centre for Working Environment and Human Factors and the TU Dortmund. The aim of the RTG is the development and application of biostatistical methods for the analysis of high-dimensional data for modeling and risk assessment in toxicology. Doctoral researchers acquire knowledge in toxicology and the ability to develop and apply statistical methods for questions in pharmacological and environmental toxicology. In toxicology, innovative statistical methods are required to optimally exploit the ever-growing, heterogeneous, molecular flood of data for adequate modeling and risk prediction. In addition to conventional one-dimensional dose-response models, more complex models need to be developed. High-dimensional omics data are used in modeling both as an interaction factor for toxicological exposure and as a target.
Novel health care strategies for melanoma in children, adolescents and young adults (MELCAYA)
In childhood, adolescence and young adults (CAYA), melanoma is under-studied and non-existing tailored clinical guidelines and standardized approaches lead to a very low diagnostic accuracy. The MELCAYA project aims to understand risk factors and determinants of melanoma to improve the prevention, diagnosis and prognosis of melanomas in CAYAs. The Krutmann lab contributes together with the Schikowski lab to the identification of risk factors, exposomics and genetic susceptibility of melanoma in CAYAs. The funding is provided within the HORIZON-MISS-2021-Cancer-02-03 funding scheme (2022-2028).
Taizhou project (China)
The project “Air pollution exposure, its interaction with genes and the role of systemic inflammation on skin-related outcomes in an elderly Chinese population”, is a collaboration with the Max Planck-CAS Paul Gerson Unna Research Group on Dermatogenomics in Shanghai, China (cooperation partner: Dr. Sijia Wang). The study investigates the long-term effects of air pollution exposure on skin-related outcomes in an elderly Chinese population. The focus is on gene-air pollution interactions to identify possible susceptible groups and additionally focus on systemic inflammation and its role in skin-related outcomes after long-term air pollution exposure. This project is in close collaboration with the working group Schikowski. The external collaboration partners are Prof. Li Jin, Prof. Theresa Wang and Prof. Haidong Kan (Fudan University, Shanghai, China). The project is supported by the joint IUF-PICB research project as well as industrial funding.
CASAI cohort (India)
The project “Climate, air pollution and skin aging in Indian women (CASAI)”, is a collaboration with the Centre for Environmental Science and Engineering, Indian Institute of Technology (IIT) Bombay, Mumbai, India (cooperation partner: Dr. Harish Phuleria). The study investigates the long-term effects of climate and air pollution exposure on extrinsic skin aging signs in Indian women above the age of 20 years. The study focuses on the interactive effect of climate and air pollution together which have been identified as important environmental factors to induce premature skin aging signs. India, with diverse climatic conditions, extreme temperatures and high air pollution exposure, provides an opportunity to discover extrinsic skin aging signs in a population which is ethnically and socioeconomically different from Western and other Asian countries. The cohort was established between the years 2018-2020 in three major metropolitan cities of India: Delhi, Mumbai, and Bangalore, and examined 1500 participants in the baseline investigation. This project is in close collaboration with the working group Schikowski and is supported by funding from Amway.
IUF internal:
WG Esser
WG Koch
WG Schikowski
WG Schins
WG von Mikecz
WG Haarmann-Stemmann
WG Ventura
LG Weighardt
Core Unit GEMD (Rossi)
National:
Prof. Bernhard Homey, Dep. of Dermatology, University Hospital Düsseldorf
Prof. Karin Loser, Dep. of Immunology, University Oldenburg
Priv.-Doz. Dr. Stephan Meller, Dep. of Dermatology, University Hospital Düsseldorf
Prof. Sven Meuth, Dep. of Neurology, University Hospital Düsseldorf
Prof. Thomas Tüting, Dep. of Dermatology, University Magdeburg
International:
Prof. Jean-Marc Egly, University of Strasbourg, France
Prof. Akimichi Morita, Nagoya City University, Nagoya, Japan
Prof. Thierry Passeron, Côte d'Azur University, Centre Hospitalier Universitaire de Nice, Department of Dermatology, France
Prof. Henry Lim, Department of Dermatology, Henry Ford Health, Detroit, Michigan, USA
Prof. Jiucun Wang, Fudan University, Department of Anthropology and Human Genetics, Shanghai, China
Prof. Sijia Wang, PICB, Shanghai, China
The Krutmann research group is together with S.Grether-Beck in charge of the IUF’s "Human in vivo studies".
PhD student
Technical assistance
- Selina Dangeleit, BTA
- Maren Knechten, BTA
- Elisabeth Springer, BTA
- Ingo Uthe, BTA



