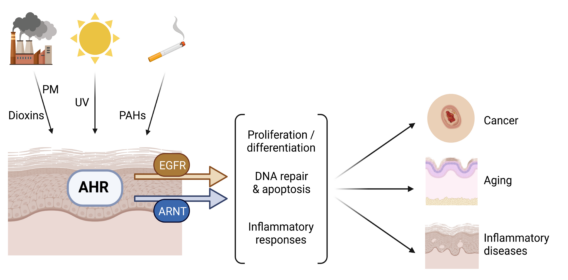
Our research group investigates the biological functions of the aryl hydrocarbon receptor (AHR) signaling pathway in skin exposed to environmental stressors. The main focus of the research is to elucidate new functions of the AHR in the stress response of cutaneous cells, in particular epidermal keratinocytes, exposed to ultraviolet radiation (UV) and ubiquitous environmental pollutants, such as polycyclic aromatic hydrocarbons (PAHs) and dioxins. Our overall aim is to identify novel molecular targets for the development of preventive and therapeutic measures against environmentally induced skin diseases. For the first time, we have identified the AHR to critically contribute to skin photocarcinogenesis in mice by repressing both, nucleotide excision repair and apoptosis in UVB-irradiated keratinocytes. Currently, we assess the potential impact of a cross-talk between different exposomal factors on adaptive and maladaptive stress responses in the skin. In the context of skin aging and carcinogenesis, the group collaborates with the Krutmann laboratory to compare the outcome of single UVA and UVB irradiation protocols versus sequential and simultaneous exposure. By combining chemical treatment with UV exposure protocols, our group investigates whether a modulation of DNA repair by AHR agonists has an impact on UVB-initiated skin carcinogenesis in mice. Just recently, we found that PAHs but not dioxin-like compounds, stimulate the expression of aldo-keto reductase (AKR) 1C3 and the associated 11-ketoreduction of prostaglandin D2 via a non-canonical AHR signaling pathway. The resulting metabolite, 9,11-prostaglandin F2, may contribute to the worsening of atopic dermatitis, by stimulating and maintaining Th2-driven inflammatory reactions. In collaboration with the Schikowski group, we are currently investigating whether a gain-of-function polymorphism in the coding region of the AKR1C3 gene is associated with an enhanced individual susceptibility to airborne particulate matter-associated atopic dermatitis.

Head of working group: Thomas Haarmann-Stemmann
Role of the aryl hydrocarbon receptor in ultraviolet-B radiation-induced DNA damage responses and skin carcinogenesis
In this DFG-funded research project we investigate the mechanism by which ligand-based AHR activation influences nucleotide excision repair. By conducting in vitro experiments as well as a carcinogenesis studies we test the hypothesis that exposure-relevant AHR agonists, such as PAHs, act co-carcinogenic and enforce the development of UVB-initiated squamous cell carcinomas (SCC). The results of these studies are in particular of clinical relevance for individuals that are daily exposed to both stress factors and thus are at high risk for SCC development, such as roadmen and roofers. An additional facet of this project is the potential anti-apoptotic role of AHR in keratinocyte-derived tumor cells. By conducting various in vitro as well as in vivo studies, we will assess whether an inhibition of AHR sensitizes cutaneous cancer cells to genotoxic drugs. In case this approach is feasible, it may allow to refine topical chemotherapy by reducing respective treatment doses and times.
Interaction of different UV wavelengths present in sunlight
In close collaboration with the working group of Jean Krutmann, we are studying the interaction of different UV wavelengths present in sunlight in human keratinocytes and mice. The rationale for this project is the artificial design of the majority of photobiological studies, consisting either of an irradiation of the test model with one part of the solar spectrum (e.g., UVB light) or a sequential exposure to two or more parts (e.g., UVB first, followed by UVA). However, in contrast to this, a simultaneous exposure to two or more parts of the solar spectrum is way closer to the human exposure situation. In this project, we therefore compare the effects of single and sequential UVA and UVB irradiation with respective simultaneous exposure protocols. Amongst other parameters, the major readouts of these experiments are DNA damage, DNA repair, oxidative stress, apoptosis and skin carcinogenesis. The overall aim is to enhance our mechanistic understanding of the adaptive and maladaptive cutaneous events that are induced by solar UV radiation. The outcome of this project may help to improve measures for skin photoprotection.
Identification of the epidermal growth factor receptor (EGFR) as a molecular target for ubiquitous organic environmental pollutants
In this project we assess the potential interaction of dioxins, polychlorinated biphenyls (PCB) and structurally-related halogenated aromatic hydrocarbons with the extracellular domain (ECD) of the EGFR. We have previously identified amino acid residues located in the EGFR ECD that are involved in the binding of PCB congener 126 and are responsible for its anti-proliferative effect on epidermal keratinocytes. In the current project, we focus on the identification of EGFR-binding environmental pollutants and the molecular and cellular characterization of the associated adverse cutaneous effects. We believe that the inhibition of growth factor-induced EGFR activation and downstream signal transduction is a so far unknown mode of action of numerous exposure-relevant pollutants. A deeper understanding of the underlying mechanisms and consequences is thus key to understand and prevent the toxicity induced by these compounds. This research project is funded by the Jürgen Manchot Foundation.
Impact of polycyclic aromatic hydrocarbons on cutaneous prostaglandin D2 metabolism and associated inflammatory responses
Background of this research project is our previous observation that the expression of the prostaglandin D2 (PGD2)-reducing aldo-keto reductase (AKR) 1C3 is induced in response to PAHs via a non-canonical AHR signaling pathway. The NADPH-dependent oxidoreductase AKR1C3 catalyzes the 11-ketoreduction of PGD2, which is released by degranulating mast cells and stimulates Th2-driven inflammatory responses in the skin, to 9α,11β-PGF2. This metabolite is also bioactive but in contrast to the parental PGD2 metabolically stable. In the current project, we want to understand the functional and clinical relevance of the AHR-AKR1C3 axis and the associated alterations in PGD2 metabolism for the aggravation of Th2-driven atopic dermatitis by airborne PAHs and PAH-rich particulate matter. To this aim, we are conducting mechanistic studies in keratinocytes in 2D and 3D culture and human ex vivo skin. The clinical relevance of AKR1C3 for pollution-mediated effects on atopic dermatitis patients is investigated in close collaboration with Tamara Schikowski and her working group. This project is funded by the Jürgen Manchot Foundation.
IUF internal:
WG Esser
WG Krutmann
WG Schikowski
Core Unit GEMD (Rossi)
International:
Julien Dairou, University of Paris, France
Rodolfo Lavilla, University of Barcelona, Spain
Motoki Nakamura, Nagoya City University, Japan
Christoph F.A. Vogel, University of California, Davis, CA, USA
Postdoc
- Stephanie Binder (joint project with Andrea Rossi)
- Hatice Genc
- Katharina Rolfes
PhD students
Master students
Technical assistance
- Ragnhild Wirth, BTA



